WHO 1999: The Problem of Counterfeited Medicines
WHO counterfeit medicine database
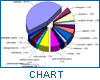
Since the Nairobi meeting, public awareness of the problem of counterfeit medicines has been growing. Both government authorities and manufacturers have been giving attention to its prevention, and WHO has been receiving reports related to counterfeit drugs. According to this database, the problem of counterfeit drugs is known to involve both developed and developing countries. For instance, between 1982 and April 1999, 771 confidential and public reports relating to such drugs were received by WHO.The geographical origin of these reports is shown in Figure 1.
The public reports received include some anecdotal accounts which were published in journals. The confidential reports were received from national authorities for regulatory Information purposes. Although most of the reports are not confirmed, and bias is likely, they provide some insight into the problem.
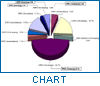
The pharmaco-therapeutic classes of the cases reported are shown in Figure 2, which indicates that the majority of detected counterfeit products in the database are die "life-saving" drugs, i.e. antibiotics.
In developing countries, die two top-ranking classes of medicines reported to be counterfeited are anti-infectives and anti-parasites, whereas in industrialized countries anabolic steroids and dermatological products accounted. for die majority of counterfeit products.
Out of the 771 cases reported, indications about die quality of die active ingredients contained were supplied only for 325 cases. Of these, about 59% contained no active ingredients, 7% contained die correct amount of active ingredients, 17% contained die incorrect amount of active ingredients and 16% contained different active ingredients. No indication was given about die quality of die active ingredients of die remaining 58% of die case reports.
To further supplement the database, in May 1996 the WHO Division of Drug Management and Policies (DMP) sent a questionnaire to the information officers* of Member States, the six WHO regional offices and other organizations to obtain information on counterfeit drugs. Of the 46 countries that had responded to the questionnaire as of March 1997, 41% recognized the existence of problems of counterfeit drugs in their domain.
Previous country studies related to counterfeit medicines
In addition to the WHO database, there are reports on studies carried out in different countries to assess the quality of pharmaceutical products available on the market and to establish whether they are counterfeit. For instance, between 1990 and 1993 the Drug Quality Control Centre (DQCC) of the Lao People‘s Democratic Republic (LPDR) carried out three studies.15
In the first study 502 samples were examined. Of these, 247 were examined in relation to drug registration and 168 in relation to post-marketing monitoring. The results of laboratory tests showed 87 (17%) of the samples to be substandard.
In the second study, the DQCC tested 112 samples collected from different provinces, 37 (33%) of which failed to meet quality standards. Gut of the 37 samples, 18 (49%) contained less than 50% of the amount of active ingredients claimed. In the third study, 25 samples were analysed and the results indicated that the amount of active ingredients contained ranged from 0% to 95%. All products without active ingredients were classified as counterfeit.
A similar study carried out in 1995 on samples of pharmaceutical products taken from the markets of different provinces of the LPDR showed several of the products to be substandard. Some products contained no active ingredient
A WHO mission that visited Viet Nam‘7 reported that counterfeit drugs prevail nationwide, including in the rural areas. The products counterfeited were antibiotics, vitamins, antimalarials and oral corticosteroids. Laboratory tests revealed three categories of fake drugs — those with no active ingredients, substandard products and those containing wrong ingredients.
A survey conducted by the National Institute of Drug Quality Control of Viet Nam over a period of two years (1990-1991) reported that out of 25,000 samples collected from 20 provinces, 1771 (7%) failed on testing.‘ The types of counterfeiting were reported to include the following: wrong ingredients, without active ingredients, containing incorrect quantities of active ingredients, and with fake packaging.
Similarly, nationwide quality monitoring was carried out in 1995.‘~ Gut of 31,123 samples tested, 1703 (6%) samples failed to pass quality tests. Of these, 166 (0.5%) contained wrong ingredients or no active ingredients.
In Africa, a survey of the quality of drugs moving in the markets of three countries was carried out between 1991 and 1993.20 Some 519 samples were collected from private, public and nongovernmental drug outlets as well as from illegal markets in three countries. The quality of 429 samples was assessed by sending them to independent laboratories for testing. A total of 352 were found to be in conformity with the specifications, while the remaining 77 (18%) were found to be substandard. Of the 77 samples that failed the tests, 16 contained no active ingredients.
In the Philippines, a study called Project Philcap (Philippines Counterfeit Action Programme) was conducted in 1995.21 A total of 1359 samples were bought from 473 drug stores and tested. Of the samples, approximately 8% were reported to be counterfeit. About 11% of the drug stores visited were found to be dealing in counterfeit products.
According to the WHO definition of counterfeit drugs, the following actions need to be carried out to establish whether a given pharmaceutical product 15 counterfeit:
- the identity and content of the active ingredient(s) in the product should be determined by laboratory tests;
- the source of the product, the manufacturer and the country of manufacture have to be confirmed through investigation;
- in circumstances where the product is found to be from a genuine source (manufacturer and country of manufacture) but contains no active ingredient(s), or contains the wrong ingredient(s), or contains the incorrect quantity(ies) of active ingredient(s), an investigation should be carried out to establish whether this is deliberate or not.
This investigation should be carried out by contacting the drug regulatory authority (DRA) of the country of manufacture and the manufacturer of the product.
The findings reported in the above-mentioned studies are based solely on results of laboratory tests of samples of products. None of them indicated the types of investigations carried out to establish whether the products were counterfeit. It may be possible to classify a product as counterfeit if laboratory tests show that it does not contain any active ingredient(s) or contains wrong ingredient(s). But problems will arise if laboratory tests show that the product under study contains incorrect quantity(ies) of the active ingredient(s) or correct quantity(ies) of the active ingredient(s). In this case, depending on the genuineness of the source, manufacturer and country of manufacture, the product may be classified as a counterfeit, genuine-substandard or genuine-correct quality product.
Source:
Counterfeit and substandard drugs in Myanmart and Vietnam, Eshetu
Wondemagenchu, EDM Research Series No. 29, WHO, Geneva, 1999